Caco3 Ionic Or Covalent. Is caco3 a covalent network solid? The carbon has one double bond and two single bonds.
A bond shaped between nonmetal atoms is covalent. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. Calcium chloride is highly soluble in water owing to its ionic nature.
A Bond Shaped Between A Metallic And Nonmetal Atom Is Ionic.
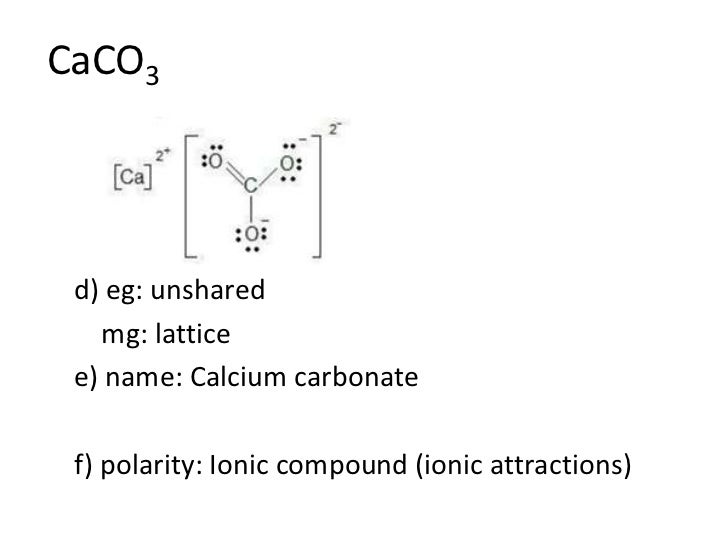
Calcium carbonate has both ionic and covalent bonds. Another example of an ionic compound is marble (caco3) (also known as calcium carbonate). The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic.
Calcium Carbonate (Caco3), Essentially, Is An Ionic Compound Having The Bivalent Calcium And Carbonate Ions.
Calcium carbonate (caco3), essentially, is an ionic compound having the bivalent calcium and carbonate ions. Is caco3 an ionic or molecular bond ? Within the carbonate ion, there are covalent bonds.) how do the names covalent compound differ from names of.
Secondly, Due To The Large Difference Of Electronegativities (∆E=2.4), The Calcium Atom Easily Loses Two Of Its Outermost Shell Electrons, Which Are In Turn, Gained By The Oxygen Atom.
It is known as polyatomic ion. This charge attracts and keeps the ca (which is actually +2). Calcium oxide is considered an ionic compound as it comprises a metal (calcium) and a nonmetal (oxygen).
Caco3 Is Ionic Or Covalent.
What is structure of cac2? To tell if cuco3 (copper (ii) carbonate) is ionic or covalent (also called molecular) we look at the periodic table that and see that cu is a metal and co3 i. According to fazan's rule, a compound is covalent when it contain small cation, large anion and high charge.
To Tell If Caco3 (Calcium Carbonate) Is Ionic Or Covalent (Also Called Molecular) We Look At The Periodic Table That And See That Ca Is A Metal And Co3 Is A.
But the carbonate anion is a polyatomic species. It is also an ionic compound since its ionic bonds are formed with atoms of a metal and a nonmetal. But the carbonate anion is a polyatomic species.
Related Posts
- Definition Of Nonpolar Covalent BondsDefinition Of Nonpolar Covalent Bonds. Medical dictionary, © 2009 farlex and partners. Bonds that are partly ionic are called polar covalent bonds.Ho ...
- Which Of The Following Contains Both Ionic And Covalent BondsWhich Of The Following Contains Both Ionic And Covalent Bonds. By definition, an ionic bond is between a metal and a nonmetal, and a covalent bond is ...
- Hbr Ionic Or CovalentHbr Ionic Or Covalent. Finally, if the bond is between.5 and 2 is a polar covalent bond. Actually the difference in electronegativity between h (2.20 ...
- Mgcl2 Ionic BondMgcl2 Ionic Bond. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; Beside above, doe ...
- Sodium Chloride Ionic Or CovalentSodium Chloride Ionic Or Covalent. Is nacl ionic or covalent? Ionic bonds are electrostatic interactions formed between ions of opposite charges.chem ...
- One Carbon Atom Can Form How Many Covalent BondsOne Carbon Atom Can Form How Many Covalent Bonds. One carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons b ...
- Is Cacl2 Ionic Or MolecularIs Cacl2 Ionic Or Molecular. Since h2o2 h 2 o 2 involves covalent bonding, this is considered as a molecular compound or substancce. The hydrogen and ...



