Dichlorine Monoxide Polar. Naming acids introduction vapor pressures is mostly a function of intermolecular forces. Dichlorine monoxide has been shown to be effective in bleaching of pulp (qv) and textiles.
Naming acids introduction vapor pressures is mostly a function of intermolecular forces. A scientist's guess about how something works b. Dispersion, dipole (because it is made of polar molecules) carbon disulfide.
Dichlorine Monoxide Has Been Used As An Intermediate In The Manufacture Of Calcium Hypochlorite And In Sterilization For Space Applications.
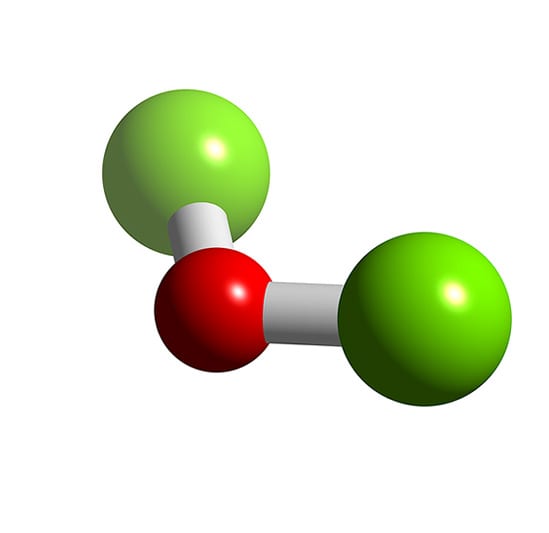
In saturated organic compounds, all the bonds between carbon atoms are called? Molecular geometry is physical representation of the relation between elements in a compound. It contains two chlorine atoms and one oxygen atom and we will learn how to draw the lewis structure of cl 2 o step by step in this tutorial.
A Scientist's Guess About How Something Works B.
The electronegativity values for oxygen and chlorine are 3.44 and 3.16 where their difference is 0.28. What type of bond is dichlorine monoxide? The results of an experiment obtained.
Carbon Monoxide Is A Hetero Nuclear Diatomic Molecule.
Reason been because both c2hcl. Dispersion, dipole (because it is made of polar molecules) carbon disulfide. It has a molecular weight of 86.9054 g/mol.
Polar In Chemistry, Polarity Is A Separation Of Electric Charge Leading To A Molecule Or Its Chemical Groups Having An Electric Dipole Or Multipole Moment.
The lewis structure for co has 10 valence electrons. Dichlorine monoxide, is an inorganic compound with the molecular formula cl 2 o. A teacher walks into the classroom and says if only yesterday was tomorrow today would have been a saturday which day did the teacher make this statement?
Both Are Polar Molecu… View The Full Answer Transcribed Image Text :
In older literature it is often referred to as chlorine monoxide, which can be a source of confusion as that name now refers to the neutral. Cl2o ( dichlorine monoxide ) is polar i'll tell you the polar or nonpolar list below. Naming acids introduction vapor pressures is mostly a function of intermolecular forces.