Electron Configuration Of Ru 3. The ruthenium nanoparticles showed a marked increase of the average diameter with increasing pyrolysis temperature, ca. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals.
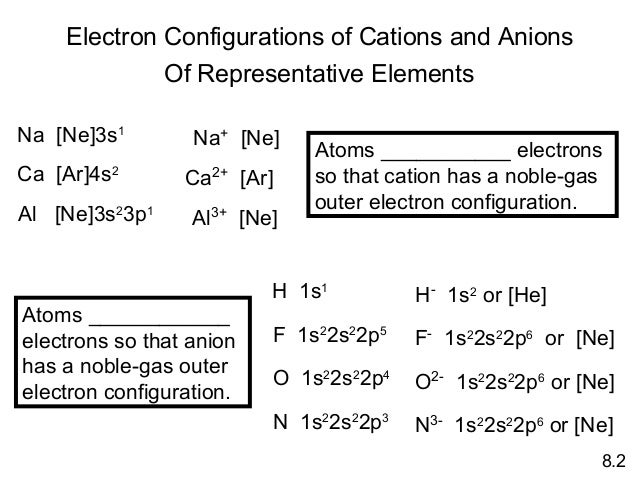
For example ti(z = 22) is in period 4 so that n = 4, the first 18 electrons have the same configuration of ar at the end of. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties.
There Are 118 Elements In The Periodic Table.
To prevent electron entering the 3d orbitals. Geolocation tracking, historical tracking, fleet configuration. For example ti(z = 22) is in period 4 so that n = 4, the first 18 electrons have the same configuration of ar at the end of.
The Electron Configuration Is The Distribution Of Electrons Of An Atom Or Molecule (Or Other Physical Structure) In Atomic Or Molecular Orbitals.
The ground state electronic configurations of the outer orbitals of transition elements are given in table 8.1. The periodic table is a tabular display of the chemical elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. The neutron is a subatomic particle, symbol n or n 0, which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton.protons and neutrons constitute the nuclei of atoms.since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one atomic mass unit, they are both referred to as nucleons.
Each Element Has A Unique Atomic Structure That Is Influenced By Its Electronic Configuration, Which Is The Distribution Of Electrons Across Different Orbitals Of An Atom.
Similarly in case of cu, the configuration is 3d104s1 and not 3d94s2. Electron configuration chart for all elements in the periodic table. An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state.
The Ruthenium Nanoparticles Showed A Marked Increase Of The Average Diameter With Increasing Pyrolysis Temperature, Ca.
O h point group contains 3 c 4, 4 c 3, 9 c 2, 4 s 6, 3 s 4, 3 σ h, 6 σ d and a centre of inversion inversion operation is a reflection through the centre of the molecule. In this case, the centre of the molecule is the sulfur atom. Ru mo nb tc cr rh v.
Bring Your Iot Product From Concept To Market With A Team Of Iot Experts.
1) ionization potentials (see li) 2) electron affinities (see cl & f) 3) ground state configurations (e.g., c) let’s look again at the ordering of atomic orbitals, paying particular attention to the 3d and 4s in transition metals.
Related Posts
- Ne Electron ConfigurationNe Electron Configuration. Electron configuration the periodic table is a tabular display of the chemical elements organized on the basis of their at ...
- Zinc Noble Gas ConfigurationZinc Noble Gas Configuration. It can also be written by writing a noble gas (argon) and then writing the remaining electrons as, The zn belongs to pe ...
- Abbreviated Electron Configuration For LeadAbbreviated Electron Configuration For Lead. Electron configuration of boron (b) [he] 2s 2 2p 1: When electron configurations become too long and ted ...
- Socl2 Molecular Geometry And Electron Pair GeometrySocl2 Molecular Geometry And Electron Pair Geometry. The molecular geometry of socl2 is trigonal pyramidal and its electron geometry is tetrahedral. ...
- Condensed Electron ConfigurationCondensed Electron Configuration. The condensed electron configuration includes the symbol for the nearest, smaller noble gas in square brackets and ...
- Mercury Element Electron ConfigurationMercury Element Electron Configuration. A complete explanation of mercury's extreme volatility delves deep into the realm of quantum physics, bu ...
- Electron Dot Structure For No3Electron Dot Structure For No3. This is the best answer based on feedback and ratings. This is in part to their high solubility in water.Calculating ...

