Molecular Geometry Bf4. Asked by jasen runte give me. 10 = linear, 11 = trigonal planar, 12 = bent,.
With the reference of chemistry, ‘trigonal planar’ is a model with three atoms around one atom in the middle. Experts are tested by chegg as specialists in their subject area. The molecular geometry of sf4 according to its molecular formula and hybridization is trigonal bipyramidal.
What Is The Molecular Geometry For Bh4?
We review their content and use your feedback to keep the quality high. 10 = linear, 11 = trigonal planar, 12 = bent,. Asked by jasen runte give me.
The Compound Behaves Differently In Different States Of Matter.
Again, an extra electron shown in red that is not present in the ground state configurations will provide the negative charge (shown in red) answer link This seems more likely to me. If i decided to study chemistry at home what kind of preparations i must take, to be able to read books in chemistry?
)Hybridization Changes And The Bond Angle Increases.
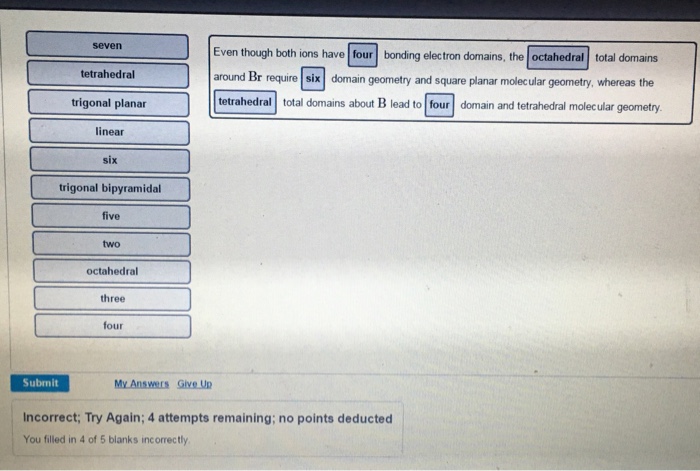
2 = sp2 3 = sp. Boron has 3 valence electrons, and each of the four fluorides contributes one electron to each covalent bond The molecular geometry of this molecule is square planar as this how the different lone pairs of both atoms adjust with one another.
With The Reference Of Chemistry, ‘Trigonal Planar’ Is A Model With Three Atoms Around One Atom In The Middle.
The molecular geometry of sf4 according to its molecular formula and hybridization is trigonal bipyramidal. Which molecule or ion does not have a tetrahedral molecular geometry? The geometry of molecule of bf3 is ‘trigonal planar.’.
It’s Like Peripheral Atoms All In One Plane, As All Three Of Them Are Similar With The 120° Bond Angles On Each That Makes Them An Equilateral.
Trending questions what's the most outdated thing you still use today? Among the different configuration, brf4 is a molecular structure consisting of bromine and fluorine. Boron has 3 valence electrons, and each of the four fluorides contributes one electron to each covalent bond.
Related Posts
- Is Cacl2 Ionic Or MolecularIs Cacl2 Ionic Or Molecular. Since h2o2 h 2 o 2 involves covalent bonding, this is considered as a molecular compound or substancce. The hydrogen and ...
- Sodium Chloride Ionic Or MolecularSodium Chloride Ionic Or Molecular. Or through the sharing of. In the gas phase, hcl is a polar covalent molecule, with the chlorine pulling the bond ...
- What Is Rigid Motion In GeometryWhat Is Rigid Motion In Geometry. Rigid motion is otherwise known as a rigid transformation and occurs when a point or object is moved, but the size ...
- Molecular Geometry Of Of2Molecular Geometry Of Of2. We have gone through both the uneven charge distribution and the asymmetry of oxygen difluoride. ∴ of2 formula becomes ax ...
- Of2 Electron GeometryOf2 Electron Geometry. The molecular geometry of this compound would be similar to water that entails a bent shape due to the two lone pairs of the c ...
- Cubr Molecular WeightCubr Molecular Weight. ¿es esta web útil para ti? The polymerization with 20% cubr 2 (mol/mol) showed a little big slope of molecular weight versus c ...
- The Electronic Geometry Of Nh3 Ammonia IsThe Electronic Geometry Of Nh3 Ammonia Is. What is the geometry name of nh3? What is the molecular geometry of the ammonia molecule nh3?What is the s ...




